Robert Zakar gives back to community
Food For Thought
Foods as obscure as garlic or mushrooms could be stopping you losing weight or fighting off skin or sinus problems.
Linsey Wynton tries out a new food intolerance test and wonders whether it is worthwhile.
Have you ever wondered if the foods and drinks you consume could be causing you problems?
For years I have suffered from eczema, hay fever, migraines and sinus problems. I have tried all sorts of remedies in a bid to beat these afflictions, but the steroid creams and inhalers I have been prescribed have done me little good, as have the homoepathic potions I have tried.
So, when I heard about a new food intolerance test called Alcat, I decided to try it.
The test, which was developed in America, can identify food stuff that can, apparently, exacerbate problems from skin and sinus complaints to panic attacks and even diabetes and arthritis. It can also help people who want to lose weight to identify foods which will inhibit them from doing so.
The test has recently been offered by a Brighton health and beauty salon, Saks, at the David Lloyd Club at Brighton Marina. Director Mark Woolley explained: "Some people can try all the cures in the world for particular problems and it can come down to one food they eat, like tomatoes.
"As well as helping with particular health problems, the test can help people boost their energy levels as some foods can make them feel lethargic."
The test claims to be the only one of its kind to be validated by independent scientific studies. It involves a consultation focusing on health problems and a blood test which is later sent off to a laboratory to be analysed.
At the laboratory, the blood is dispensed into separate vials containing samples of individual food stuffs including cod, grape fruit and barley. These are incubated to mimic the conditions in the body. The instruments then measure the effects of the various foods on the blood.
The results are returned within two weeks and, during a further consultation, a a therapist explains which foods should be avoided.
The only problem is that the test costs £250, meaning you need to be well-off or desperate to try it.
My consultation was with senior therapist Becky Anderson. She told me the most common problems clients had come forward with so far were irritable bowel syndrome and difficulties losing weight.
She stressed that the test did not measure food allergies, such as peanut allergy or lactose intolerance, which generally have more obvious symptoms than intolerances. She explained: "With allergies you get reactions almost immediately, like a rash or a swollen throat. Intolerances take place more internally in the body, at a slower pace, and it can be difficult to identify the food group causing them."
Foods I had a severe reaction to would need to be cut out for six months, those I had a medium reaction to would need to be cut out for three months, and those I had a mild reaction to would need to be stopped for six weeks. I would also need to cut out alcohol for at least six weeks to detoxify my body.
By reintroducing the foods one at a time, I would see if any of my ailments which may have gone, re-emerged. I could then conclude which food or foods were causing me problems.
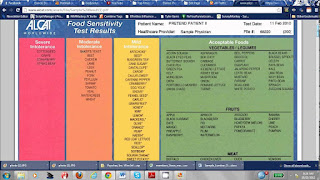
After filling out a form about my health, I had a blood sample taken by nurse Louise Amery. Two weeks later, it was time to pick up my results. I was not prepared for what I got: I appeared to be intolerant to 39 different foods, with a severe intolerance to rice and tea and a medium intolerance to potatoes, brewer's yeast and baker's yeast.
I was shocked because, not only do I consume these things regularly, cutting them out would be a nightmare: it would not be easy to live without bread, rice and potatoes for at least three months. Becky said many people's results were not as bad as mine, but unexpected things often do come up. "You have to be careful because some of the foods you may have an intolerance to will be an ingredient in other products. For example, garlic is found in chewing gum and ice cream."
Although Alcat do provide a suggested menu for different days of the week, in order to ensure you have a balanced diet, I know that if I was to stick to the results rigidly, I would not be able to eat out or cook many of my favourite meals.
In addition, Becky warned that after you cut out foods you are used to, you can suffer from a blocked nose, backache and headaches for up to a week as your body gets used to the changes.
Although I will try to cut back on some of the foods and drinks the tests recommends I cut out, I would rather have my old ailments than new eating problems.
For more details about the Alcat Test call Saks on 01273 666426.
Stability Testing For IVDs
Stability is defined as "the extent to which a product retains, within specified limits and throughout its period of storage and use (i.E., its shelf life), the same properties and characteristics that it possessed at the time of manufacture."1 According to FDA regulations, IVD reagents and consumables marketed in the U.S. Must undergo testing to determine stability.
The regulatory requirements for stability testing of IVDs are found in several places. For example, the Code of Federal Regulations (CFR) states that "the basis for such instructions (e.G., those needed to protect the stability of the product) shall be determined by reliable, meaningful, and specific test methods."2 IVDs approved by the Center for Biologics Evaluation and Research (CBER) are also subject to other CFR requirements, which state that an application for a biological license shall include "data establishing stability of the product through the dating period."3While a significant number of guidance documents directly relate to the requirements for stability testing of drugs, guidance regarding stability testing of medical devices in general and IVDs in particular is limited.1, 4, 5, 6 This article discusses the various aspects unique to stability testing of IVDs and offers suggestions on how to optimize the design of stability studies. This article also discusses effective responses to stability issues as they arise.
Design Control ElementsFor new IVDs, manufacturers should first address stability requirements during the design control stage. At this stage, IVD manufacturers should determine the device's intended use and the critical design components that must be evaluated to assure that the labeled expiration dates are met. Manufacturers should also consider user requirements and their impact on stability. IVD manufacturers should focus on user needs regarding product stability such as the following:
• Onboard stability (e.G., the maximum length of time IVDs can be loaded onto an instrument and still perform according to specifications). Shortened onboard stability requires more user intervention to replace reagents and creates delays in work output.• Labeled storage conditions. IVDs with multiple storage conditions, such as six months frozen or 24 hours thawed, require more user interaction, which could lead to errors when trying to establish the "after opening/thawing" expiration. • Preservative effectiveness for microbially controlled or sterile IVDs. The use of a preservative should control microbial growth in situations in which the user opens the device packaging multiple times during the product's life. For many IVDs, microbial contamination can result in IVD performance issues due to microbial metabolism.
The user requirements related to stability are established as design inputs and are ultimately assessed during stability studies.
Using Accelerated Study Data
Accelerated stability studies are useful for predicting the shelf life of IVDs. Such accelerated studies subject IVDs to extreme conditions—typically elevated temperatures—to the extent that the device endures significant and measurable deterioration during the testing period. Mathematical extrapolations, such as the Arrhenius equation, are then used to calculate the IVD's predicted shelf life.4 However, not all IVDs follow a predictable degradation rate. Some products will perform acceptably until they fail, in which case only real-time testing will suffice.
According to FDA's Office of In Vitro Diagnostic Device Evaluation and Safety, accelerated stability studies are acceptable in the following situations:
• Establishing preliminary claims in new products only if there is sufficient correlation to an existing product.• Supporting implementation of a change to an existing product.
The Center for Devices and Radiological Health (CDRH) recognizes the European standard EN 13640:2000, which provides guidance on not only conducting real-time and accelerated stability studies but also making calculations using the Arrhenius equation.7 Typically, CDRH requires premarket notification (510(k)) submissions to include a summary of the accelerated data, while premarket approval (PMA) submissions are expected to include the real-time study data and stability protocol. At the same time, CBER does not accept accelerated testing for either newly licensed IVDs or changes to existing IVDs. Only real-time stability data are accepted; they are also required for biologics license application (BLA) submissions, in which labeled storage and reconstituted stability data should be included.6
Overall, FDA expects all accelerated studies to be confirmed with real-time stability studies.4 However, prior to using accelerated data to support new IVDs or a significant change to existing IVDs, manufacturers should confer with the proper center at FDA about the agency's requirements regarding stability studies for specific IVD reagents.
Real-Time Stability Study Requirements
A real-time stability study includes an evaluation of those factors that ultimately affect the expiration date of IVD reagents. A number of IVD reagent stability factors should be considered when designing a stability study program (see Table I).1, 4, 5, 6While originally established as a design verification requirement, a stability study for IVD reagents has the same elements as those dictated for stability testing of drugs including the following:
• A written stability testing program designed to assess the stability characteristics of IVDs.• A stability protocol with predefined acceptance criteria that can be correlated to the label claims. • Testing multiple unique product lots. A stability study is required to use three product lots that are manufactured when the manufacturing process has been well defined and can be consistently executed.• Evaluation of each stability attribute via a statistically valid sample size and testing intervals. The sample size should be sufficient to overcome the precision of the test method used, considering the cumulative effect of all elements of the test system (i.E., individual reagents and instruments). The test intervals should be chosen so that trends may be discerned from variability of the data.4 At a minimum, stability testing should continue to one time interval past labeled expiration.• Control of material storage. For real-time stability testing, the IVD reagents should be stored under the conditions stated on the label (e.G., temperature, humidity, light protection). • Testing IVDs in the same container-closure system as the marketed product.• For IVDs that require reconstitution for use as directed in the labeling, testing IVDs for reconstitution as well as after they are reconstituted.• Use of reliable, meaningful, and specific test methods.8
This last requirement presents the greatest challenge to the design of IVD stability studies. While stability testing of drugs is accomplished through direct analytical testing of the drug itself, the typical evaluation of IVD stability involves performing the assay and determining whether the control values conform to the specifications. This technique is used regardless of the IVD component being evaluated for stability: reagent, calibrator, or control. To allow for adequate evaluation of the IVD components for stability, manufacturers should identify the control criteria for their particular system to detect and reduce the bias effect of the test system as a whole. These control criteria should consider the effects that changes in instruments, reagents, calibrators, or controls may have on the test system.
Monitoring Bias
There is a potential for failure to control the effects of bias on stability results (see Figure 1). For illustration purposes, assume the IVD component being tested for stability is a control and the acceptance criteria are such that when the assay is performed in accordance with the labeling instructions, a control value is obtained that meets the label claims. In Figure 1, the value at T3 reflects a change in the reference reagent lot used during stability testing, which introduces a bias into the stability degradation pattern. The net effect is a downward shift in the control value, thus giving more time until actual stability failure.
One method to monitor for bias is conducting parallel testing when new reference materials are introduced into a stability study. Stringent criteria should be created to assess the effects that changes in reference materials have on the stability profile. These criteria should consider the inherent variations in the test system itself, including the variation contributed by each IVD assay component as well as the instrument. Comparing the values obtained by the original reference materials with the new reference materials will detect the introduction of bias created by the new reference materials. Any shift in assay values that is outside the expected precision of the test system should prompt an investigation.
Another effective control mechanism to evaluate stability data is conducting a linear regression analysis to detect changes in the slope of the stability degradation pattern for each lot. This technique offers two unique opportunities for evaluating data:
• Detecting changes in the slope within the stability data obtained for a single lot may indicate that bias could have been introduced into the test system, which creates the potential for inaccuracy in evaluating the data.• A regression analysis of stability data allows an estimation of time until stability failure, thereby allowing a more timely response to stability issues.
Ongoing Stability Study Monitoring
Once the stability studies necessary to support the labeled expiration date of a newly designed IVD have been completed, manufacturers must then address the need for postapproval or ongoing stability studies.
Unless stipulated as a postapproval requirement, FDA does not explicitly require ongoing, or postapproval, stability study monitoring for IVDs. An FDA compliance policy guide (CPG) states that CBER-licensed IVDs generally are not required to conduct postapproval stability studies, with certain exceptions.5 While this CPG does not include CDRH-regulated IVDs within its scope, the scientific rationale regarding the need for postapproval studies in certain situations still applies; as such, CDRH-regulated IVDs should follow the same guidance.
According to the CPG, FDA investigators will cite CBER-licensed IVD manufacturers for not performing postapproval stability studies when:
• Performing the postapproval studies is required as a condition of the license.• A formulation or manufacturing change has been made.• There is a commitment to perform postapproval stability studies as part of a corrective and preventive action plan.5
Despite the limited requirements for postapproval stability studies as set forth in the CPG, ongoing stability studies are a useful and critical element of process monitoring as dictated by the CFR. One section states that, "manufacturers shall monitor production processes to ensure that a device conforms to its specifications.9 Another CFR section states that manufacturers shall analyze "quality data to identify existing and potential nonconforming product, or other quality problems."9 It is up to IVD manufacturers to evaluate and determine the need for and extent of ongoing stability monitoring, taking into consideration the risk associated with the IVD reagent as well as regulatory commitments and requirements.4 Manufacturers should be prepared to discuss with FDA the scientific rationale behind their stability study programs, including both preapproval and postapproval study designs.
Investigating Stability Failures
Once a potential stability issue has been detected during either premarket or postmarket stability studies, IVD manufacturers should initiate a timely and thorough failure investigation. In most cases, manufacturers have already released for distribution the product lots with stability issues. Nonetheless, an investigation must proceed with the knowledge that stability failures on distributed products represent a high regulatory and patient-care risk.
Stability failure investigations should include an impact analysis to determine the scope of product lots potentially affected by the problem. IVD manufacturers should place all lots on hold until they determine the scope of the stability issue. Manufacturers should report any immediate action taken to control nonconforming products, such as recalls, corrections, or removals, to FDA as required in accordance with the regulations.9, 10
IVD manufacturers can identify the product lots at risk for premature stability failure by using various techniques, including statistical tools, prior to determining the root causes. For example, assume a stability failure has occurred prior to the labeled expiration date. For purposes of this example, the decay rate for the lots at risk for stability failure is calculated to be 20 ng/ml/month, which is significantly higher than what is typically seen for this IVD.
Through linear regression and by using the upper stability specification limit at expiration, a model can be created to determine the release specification range that is most likely to fail to meet the labeled expiration date. By determining this specification at the point that intercepts the y-axis, this minimum value can then be compared with the release date for previous product lots to determine the lots most susceptible to stability failure (see Figure 2).
A root cause analysis should focus on the variables that could potentially affect product stability. Primary suspects are raw materials and manufacturing changes. For temperature-sensitive products, IVD manufacturers should examine product temperature control during manufacturing and handling. Manufacturers should also look at manufacturing activities that subject raw materials or in-process products to elevated temperatures (e.G., outside of labeled storage conditions) for any period of time, including time spent in thawing or adjusting materials to hit the target potency.
Once IVD manufacturers identify the root causes, appropriate global corrective actions must be assigned and implemented. For example, issues with raw material control may result in additional specifications. Identifying adequate corrective actions must also consider the potential gaps in the manufacturer's quality system that allowed the stability failure to occur. For example, design issues may not have adequately addressed raw material control. In addition to a raw-material specification change, the proper corrective action in this case would be to modify the design control process to include the knowledge gained from the stability failure.
After implementing the corrective actions, an evaluation of the effectiveness of the corrective actions should be conducted through verification and/or validation activities. For stability issues, this would involve activities such as placing additional product lots manufactured after the corrective action into the stability program and potentially adding test intervals to better monitor the product and/or degradation rate. The success of the corrective actions should be directly measurable. In other words, to say the measure of success of the corrective actions is that no more lots will fail stability is not adequate. The simple reason is that it would take a stability failure that equates to nonconforming products in the field to prove the corrective action was not adequate.
Conclusion
Meeting the regulatory requirements regarding stability testing of IVDs represents a challenge to IVD manufacturers. Manufacturers must consider special considerations unique to IVDs to assure that the stability study design provides accurate data to support labeled product expiration dates. A robust IVD stability program initiated as early as possible during product development, in conjunction with prompt and thorough responses to identified stability issues, assures that IVDs will continue to meet the user needs throughout their labeled expiration.
References1. Shelf Life of Medical Devices (Rockville, MD: Division of Small Manufacturers Assistance, Center for Devices and Radiological Health, 1991).
2. "In Vitro Diagnostics for Human Use," Code of Federal Regulations, 21 CFR Part 809.
3. "Licensing," Code of Federal Regulations, 21 CFR Part 601.
4. Stability Testing of In Vitro Diagnostic Reagents, EN 13640:2002 (Brussels: European Committee for Standardization, 2001).
5. Section 280.100, "Stability Requirements for Licensed In Vitro Diagnostic Products," Compliance Policy Guide (Rockville, MD: Office of Regulatory Affairs, U.S. Food and Drug Administration, 2000).
6. Guideline for the Manufacture of In Vitro Diagnostic Products (Rockville, MD: Office of Compliance, Center for Devices and Radiological Health, 1994).
7. IVD Standards Web Page (Rockville, MD: Office of In Vitro Diagnostic Device Evaluation and Safety, Center for Devices and Radiological Health, 2004 [cited 19 January 2004]); available from Internet: CLICK HERE.
8. "CGMP for the Manufacture, Processing, Packing, or Holding of Drugs and Finished Pharmaceuticals," Code of Federal Regulations, 21 CFR Part 211.
9. "Quality Systems Regulation," Code of Federal Regulations, 21 CFR Part 820.
10. "Medical Devices; Reports of Corrections and Removals," Code of Federal Regulations, 21 CFR Part 806.
Copyright ©2004 IVD Technology
Testing And Positive Cases
Ongoing Student COVID-19 Test Requirements
In order to ensure the safety of our campus, RIT is conducting weekly testing of students. Testing determines campus prevalence and identifies individual cases. Students are likely to be selected for testing multiple times this semester.
If you have completed your spring circulation requirements and are approved to be on campus, you are expected to make an appointment when invited for a COVID-19 test. You will schedule your test for a day and time that is convenient for you. Invitations are sent on Sunday and testing appointments are available Monday-Saturday. If you have signed the attestation, indicating you do not plan to circulate on campus for the spring semester, you will not be expected to participate in Tiger Testing.
Students will receive an email from tigertesting@rit.Edu that will "invite" students to make an appointment through CampusGroups; however, keep in mind that testing is mandatory.
During your appointment, you will conduct a painless, self-administered nasal swab test in front of a trained specialist. We are administering two types of tests on campus: a rapid antigen test or a PCR test. The email invitation will indicate which type of test that will be administered for the upcoming week of testing. RIT covers the cost of the test.
Alternate Testing and Exemptions
Students may choose to schedule a test off-campus if that is best for their schedule. Students testing at an off campus site will be required to complete the COVID-19 Alternate Testing and Exemption Form to notify RIT of their intended testing plans. They will then be required to submit their COVID-19 test result. Instructions will be sent to the student via email upon completion of the COVID-19 Alternate Testing and Exemption Request Form.
Students in isolation or quarantine and students who tested positive in the past 90 days will be exempt from testing.
Learn more about alternate testing and exemptions
Testing Process and Compliance
A campus-wide testing effort to identify individual cases and determine the prevalence of COVID-19 is underway. Students who are invited to participate in a Tiger Testing window are required to sign up for a testing time and show up for the test. Any students who fail to report for testing are considered non-compliant for this requirement. RIT has built a scaled response plan, which includes system access interruption (including MyCourses and Zoom) and referrals to the Student Conduct Office, which may lead to suspension or removal from housing, for those not in compliance.
Students Experiencing Symptoms
This testing is not intended for those who are experiencing COVID-19 symptoms. Students experiencing symptoms should contact the Student Health Center at 585-475-2255 or log into the RIT Wellness Portal to access the patient chat.
Tiger Testing Frequently Asked Questions


Comments
Post a Comment